Hard Biology: Epistasis
At Ginkgo Bioworks, our mission is to make biology easier to engineer. Today we're taking a closer look at epistasis, a form of biological complexity, and one way the Ginkgo foundry makes handling epistasis a little easier.
Transcript
There's a million reasons why biology is hard tech. Today we're talking about one of them: epistasis.
Sometimes biology looks easy. Like the best tech, when it works, it just works. It appears effortless. It grows itself. And when it doesn't work, people don't want to talk about it.
If you're a biologist, that means there's some hard lessons that you learn first hand. And if you don't have a bio background, the particular nature of biological hardness is like some kind of big secret. So today I'll try to give you a simulated experience of a type of challenge that comes up pretty often.
Picture yourself in the Ginkgo Bioworks foundry. You're on a metabolic engineering project for a customer that wants to make a valuable small molecule using yeast. As is usually the case, you're starting with a model of the metabolic pathway that is partial, but not complete. So the design process includes a set of candidate mutations, a list of genes that we thought might be involved in the fermentation process.
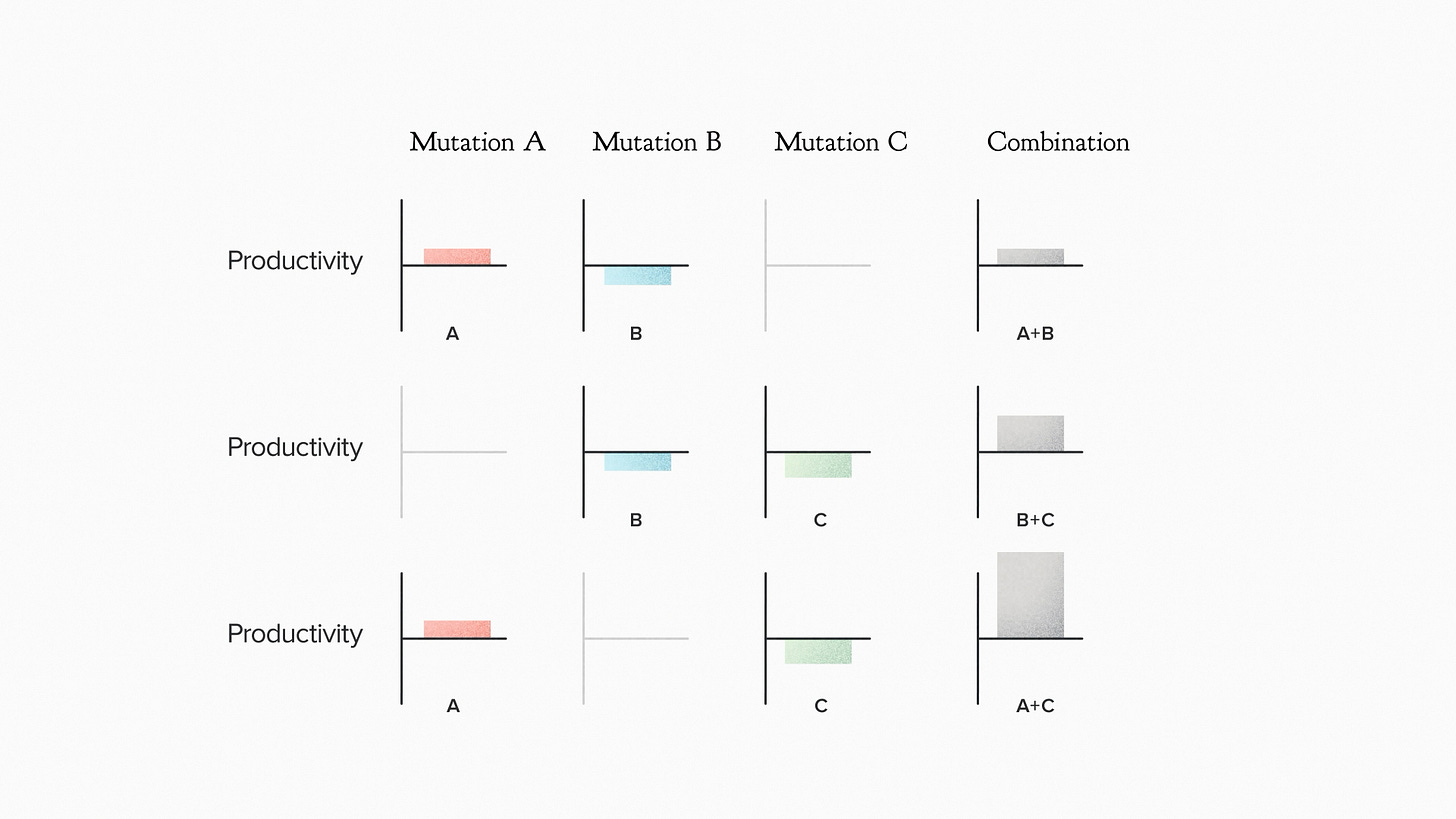
In this case, the molecule was particularly valuable and the customer wanted to really pull out all the stops to get the production as high as possible. So your team was very thorough in exploring this list of mutations combinatorially. We didn't just make each individual mutation, we made them in sets of two or three to measure the combined effects. Here's a piece of the data.
When you mutate gene A, it raises the productivity a bit. Mutating B lowers it. But when we layer B on top of A, the result looks just like A. The negative effects of B are gone.
Mutation B, alone, lowers productivity. So does mutation C. But B and C together? Productivity goes up.
One more. C lowers productivity. A alone - small increase. A + C? A huge increase. What the hell is going on here?
You, my friend, are dealing with a classic example of epistasis - the technical term for gene interaction. Because genes interact in complex and non-linear ways, a biological system can be more than the sum of its parts. Predictions, at least simple predictions, don't always work. In fact, epistasis can be defined as a deviation from simple predictions. If mutation P raises production by 3 units and mutation Q drops it by 2. The simple prediction is that P and Q together improve production by 1. If we actually make those changes and the result is -75, the difference is epistasis.
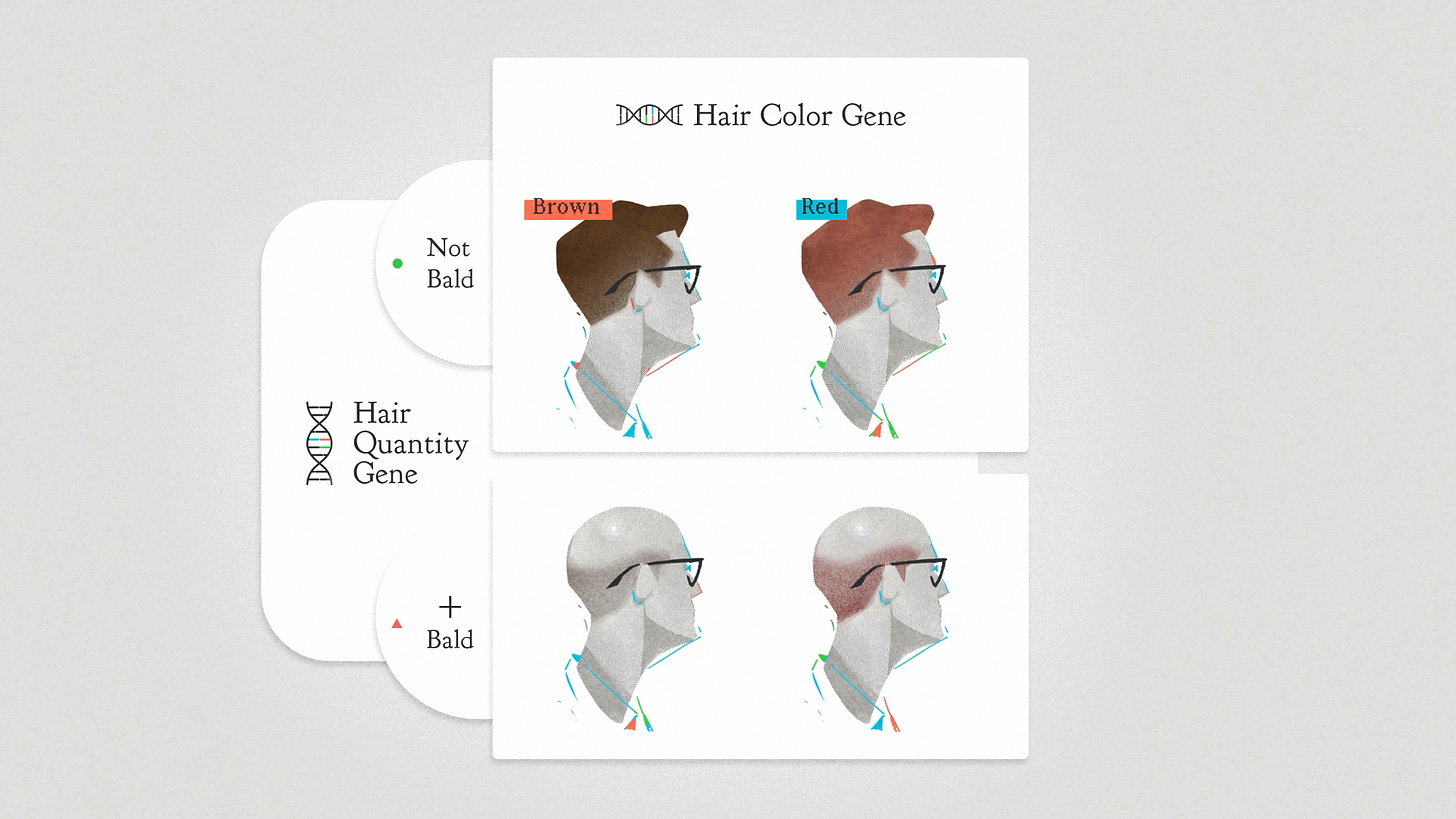
When I was a student, epistasis was part of the story of classical genetics. They start with the very simplified idea that one gene is responsible for one trait. There's a gene for red hair or for brown hair. But if there is also a gene for no hair, that changes the game. It's an epistatic interaction. The effect of the hair color gene changes depending on the context of the baldness gene.
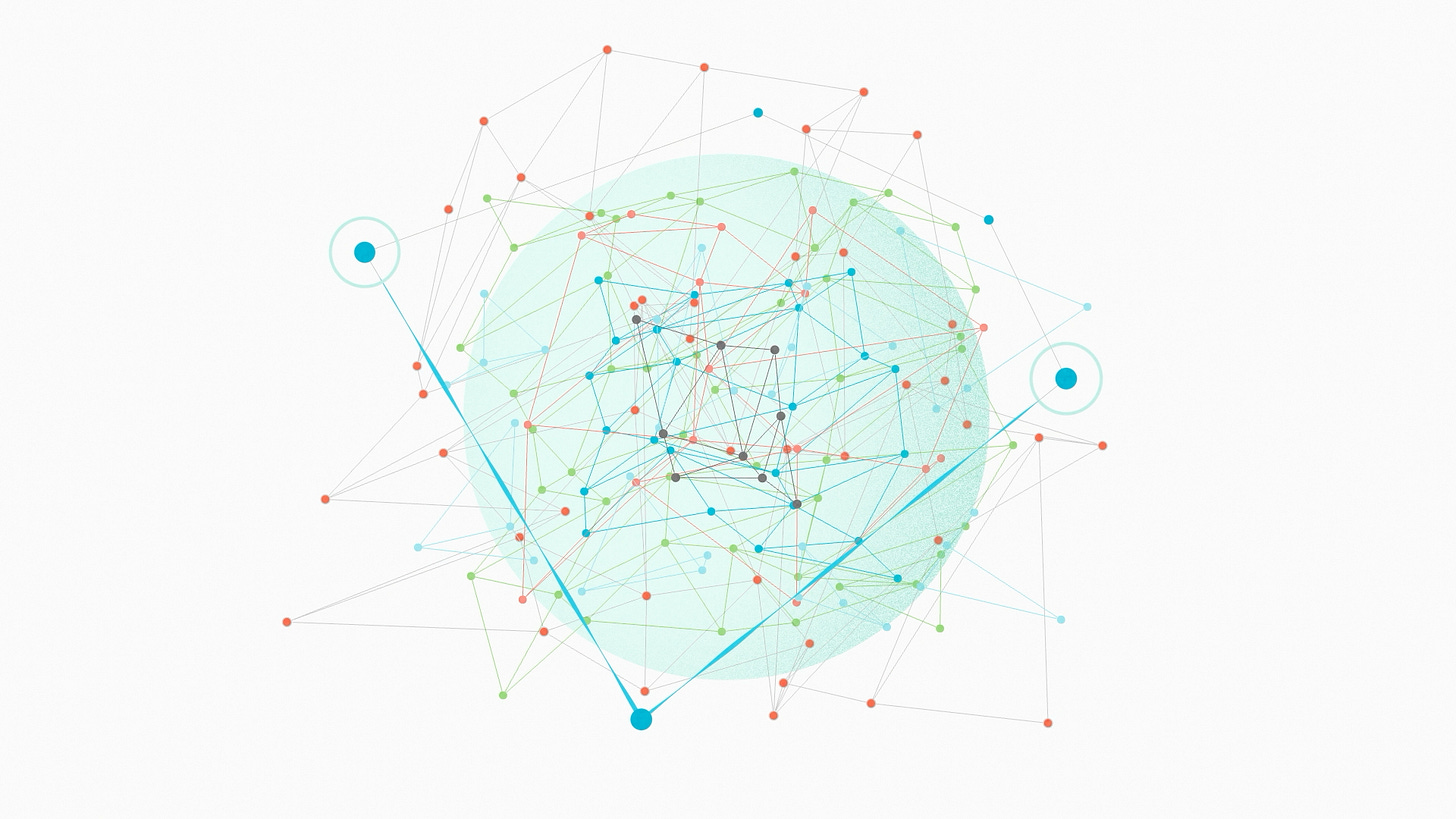
Now, in reality, there's never really one gene responsible for one trait. A cell is a complex machine, with genes organized in space and time. So instead of these very clean interaction grids, it can be more helpful to imagine a big, sprawling interaction network. This kind of diagram focuses our attention on the points of contact between different parts of the system. Each of these points of contact is a place where one gene can modify the function of another and make epistasis happen.
One strategy for dealing with epistasis is just to have a really good understanding of the system that you're working on. In other words it's a skill issue. Get good at molecular biology. Learn the genes bro.
The more of the genetic network we can fill out, the better we can predict the effects of a change in context. We can replace the simple predictions for how genes might interact with more sophisticated predictions based in finer details about how the system works. In practice, we never know all the details. But depending on the system, we often know it well enough to get some solid predictive power.
Another approach is to simply power through epistatic complexity by performing many experiments at once. We can physically make the different changes in combination and see what works. I don't have to predict how genes will interact if I can just measure it.
That's how this particular project played out. The best set of mutations for this yeast strain included A, B and C. If we had only tried them one at a time, we would have seen only their effects in isolation and we would have missed the beneficial epistasis.
We can get away with this strategy if we have the experimental power to test all those combinations efficiently. And because the number of combinatorial possibilities grows exponentially with the number of genes, we need a lot of experimental power just to push this strategy a little way.
In practice, most systems in biology want both smart design and smart scale. We use our understanding of biology to focus our exploration on the most interesting parts of the network. We use our experimental power to explore them thoroughly.
In this way, with a little luck, we can get the best of both worlds. Epistasis can still surprise us and it probably always will. But if you make the right combination of changes in a complex network, it might surprise to the upside, producing a biological system that performs better than predicted.