Metabolic Control and the Art of Optimizing Complex Things
Life, my friends, is like a biological system. Sometimes the secret to making a change is to make many changes all together. Today we're talking about engineering metabolism and why it takes a foundry to do it well.
Transcript
Most people know from personal experience that if you want to make a big impact, you usually can't get there by changing just one thing. If you want a healthier lifestyle, it takes diet, and exercise, and rest. If you want to win at sports, you need to get better at throwing the ball, catching the ball, and, you know, kicking the ball or whatever. I'm not really a sports guy.
The point is that it seems like a common feature of complex systems. They require multiplex optimization. I don't know of any theory that can explain this for sports or fitness. But at least when it comes to engineering biology, there's a pretty good mathematical framework. It's called Metabolic Control Analysis.
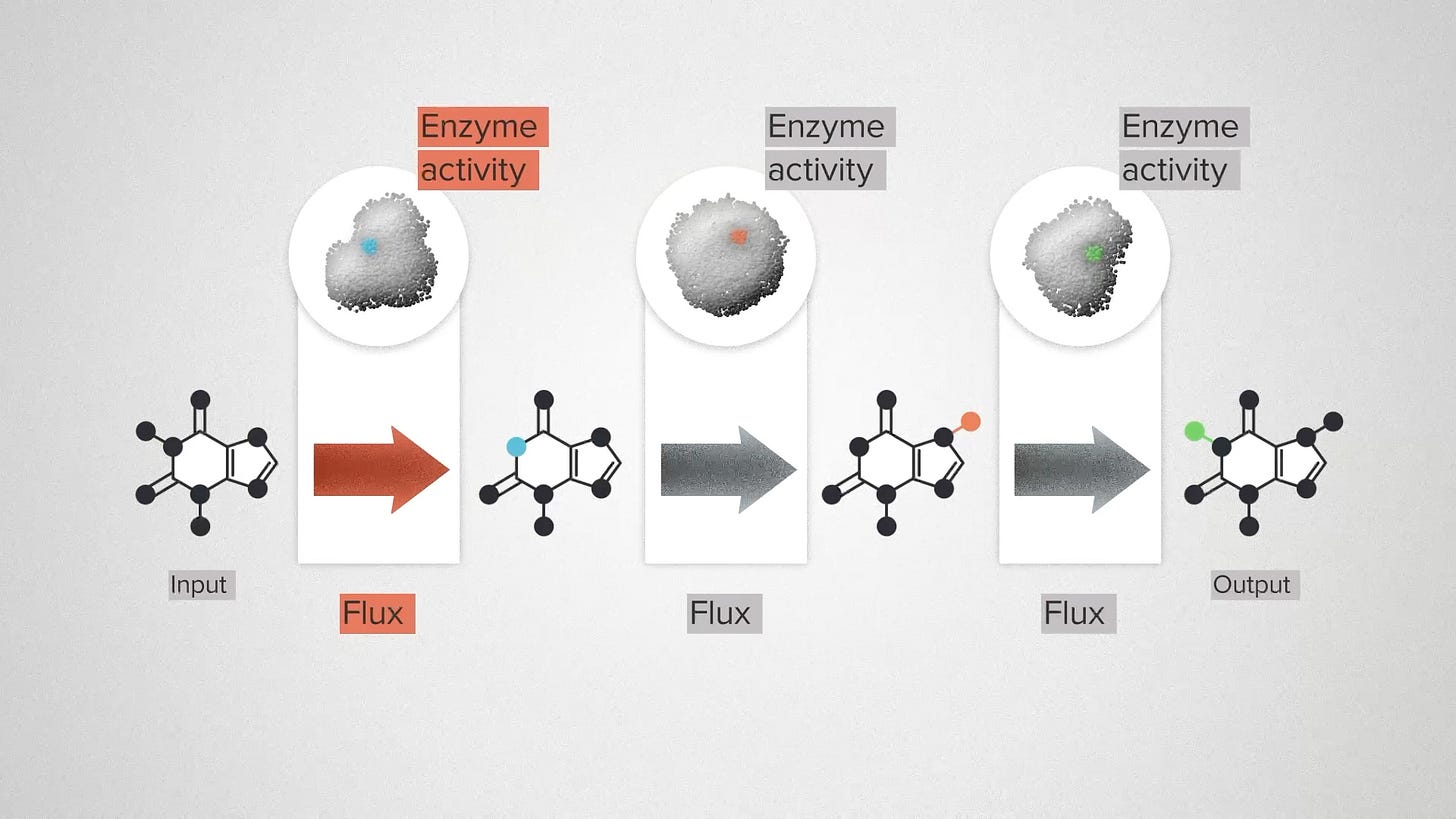
Let me give you the super speedrun version. Here we have a metabolic pathway. We're going to use an engineered organism, like yeast, to convert a cheap and sustainable chemical input into a valuable output: a flavor molecule, a fragrance, a medicine. In between, we have a series of transformation steps that are catalyzed by enzymes.
In the real world, each of these steps represents a complex chemical reaction. But we're going to ignore most of that and focus on two features. Flux is the technical term for the rate of a chemical reaction - how quickly the molecules are being transformed. Enzyme activity is a measure of how much enzyme is present. We can increase the activity by engineering the enzymes to be more efficient, or by engineering the cell to produce more of each enzyme. The challenge for a metabolic engineer is, where do I increase enzyme activity to get the most overall flux?
In the early days of biochemistry, it was a common misconception to think about this problem in terms of a rate-limiting step. The reasoning goes like this: these 3 enzymes and 3 fluxes are all linked together. The output of one is the input of the next. Therefore, the total flux through the pathway must be equal to the flux through each individual step. So far, this is all true.
You can visualize the idea as a series of pipes, with molecules flowing through them. A large pipe represents a high enzyme activity. If we have two big pipes, connected by a smaller pipe, the overall flow is constrained by the rate-limiting step.
The concept of the rate limiting step is appealing, because it suggests a simple way to optimize a complex system. All we have to do is find it, and make it go faster. Metabolic engineers would measure the activity of all of the enzymes in a pathway, identify one as the rate-limiting step, increase the activity of that enzyme, and get tiny or no improvements in the overall flux. Why didn't it work?
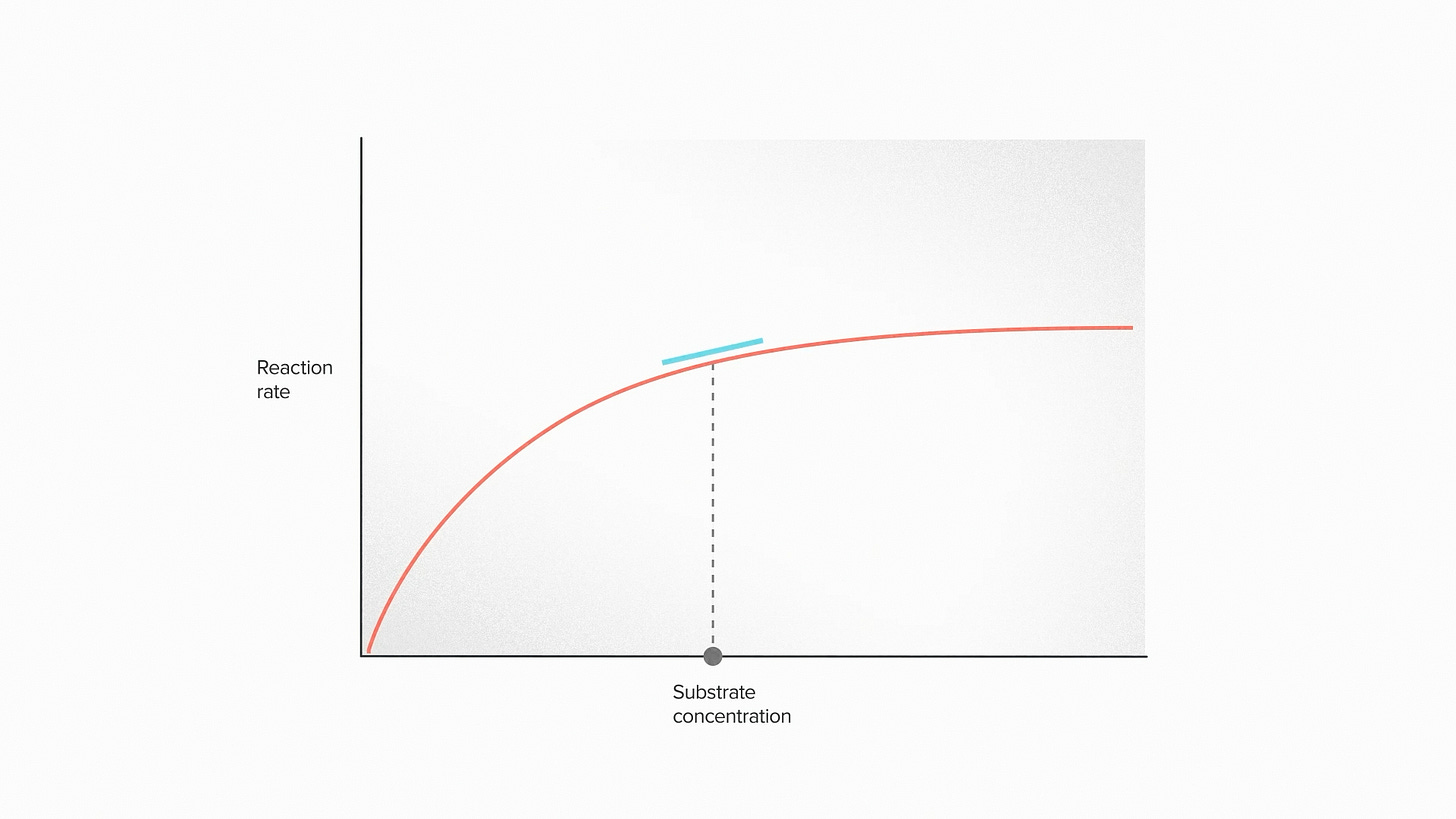
The missing concept is enzyme elasticity. This term describes the fact that an enzyme responds to it's environment. The rate of an enzymatic reaction isn't fixed, like the diameter of a pipe. It changes, for example, according to the availability of its chemical input, called the substrate.
When the concentration of the substrate drops, it takes longer to reach the enzyme and the reaction rate slows down. As the supply increases, the enzyme can work faster and faster until it reaches a point of saturation. Because of this elasticity, a fast enzyme tends to deplete its own input substrate and slow itself down. A slow enzyme can accumulate inputs and speed itself up.
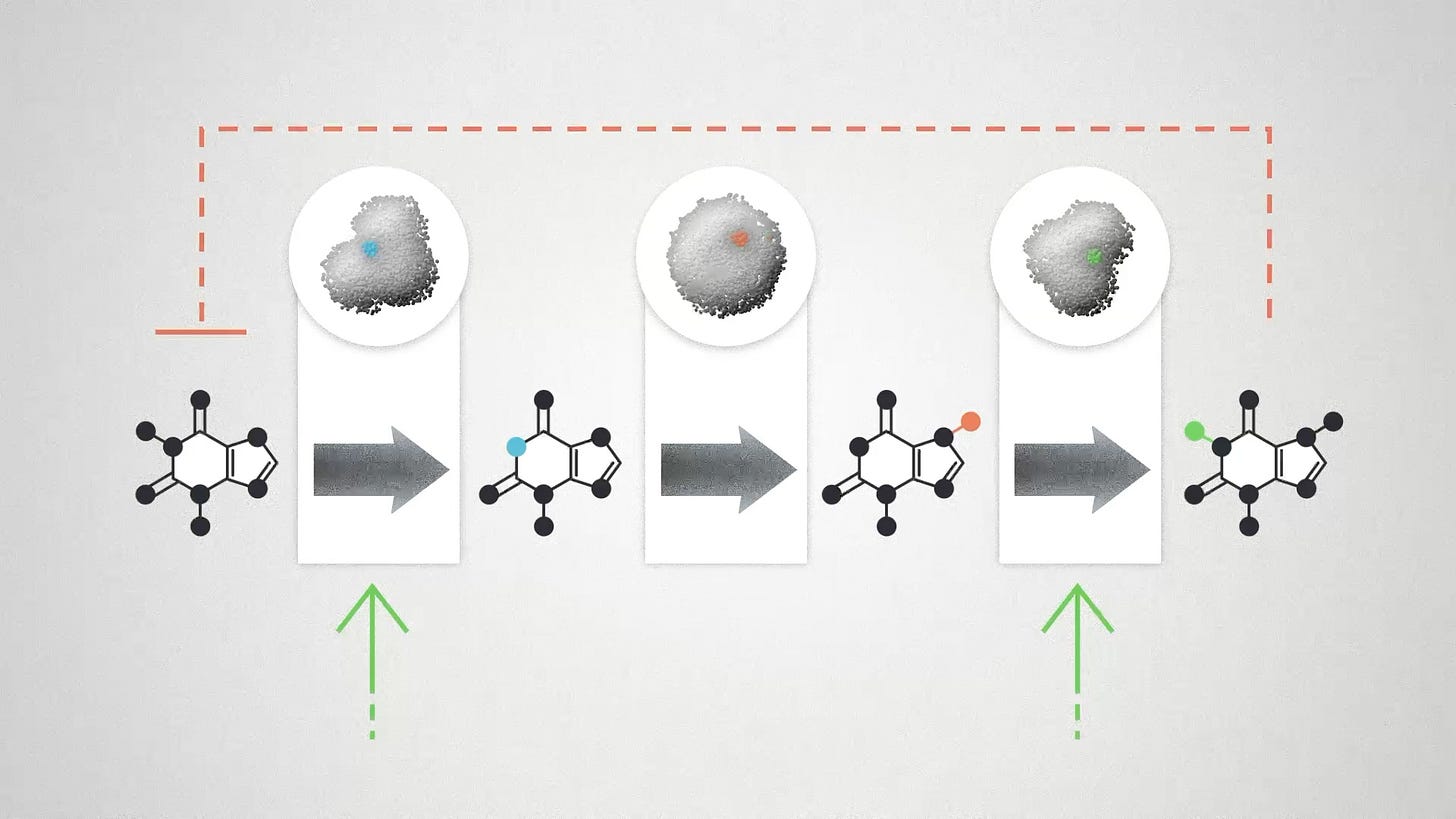
Enzyme elasticities can take other forms as well. Enzymes can respond to the concentration of other molecules in the pathway, or from regulatory signals coming from outside the pathway. The upshot is that these elasticities will tend to complicate any simple engineering strategy. Any change that you make in the enzyme activities will also affect the concentration of the molecules in the pathway. Those changes are not neutral, they are chemical signals that can work against your plans.
This complexity is why engineering biology almost always requires multiple changes. For example, if we increase the activity of an entire pathway all at once, we can improve the total flux while keeping the concentration of the input and output molecules relatively constant. We can sometimes identify places where elasticity might mess up our designs and make edits to those systems in addition to the core pathway.
Doing all that takes experimental power. It's why the foundry isn't set up to find the single rate-limiting step. We optimize an entire system in parallel and an entire bioprocess end-to-end: The pathway, and the host organism, and media, the bioreactor conditions. We push all the levers we have.
I can't prove it, but I suspect that the challenges of engineering biology are part of some general principle for solving complex problems. Maybe someday I'll have it figured out and I'll write a book about applying lessons from biology to other challenges in life. How to build your business like a cell. How to live your dreams with advice from an engineered yeast.
But for today, I'll settle for this lesson from Metabolic Control Analysis. Doing one thing well usually means doing lots of things well.